Strength of Acids
Strength of Acids: Overview
This topic describes strong and weak acids with an experimental method to determine whether the given acid is a strong acid or a weak acid. It also highlights the methods to calculate the strength of acids.
Important Questions on Strength of Acids
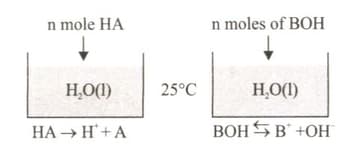
In Figure - 1 and Figure - 2, equal moles of and substances are added to the same volume of pure water at the same temperature. The equations for the solubility of these substances in water are given thereafter.
(i) The number of moles of vessel 1 equals the number of moles of in vessel 2.
(ii) The electrical conductivity of (in water) is higher than that of (in water).
(iii) The number of moles of ions in (water) is greater than the number of moles of ions in (water).
Which of the statements out of are correct?
Mineral acids are stronger acids than carboxylic acids because
Mineral acids are completely ionized in an aqueous solution.
Mineral acids are partially ionized in an aqueous solution.
Carboxylic acids are completely ionized in an aqueous solution.
Carboxylic acids are partially ionized in an aqueous solution.
Codes:
Which of the following gives the correct increasing order of acidic strength?
A sample of soil is mixed with water and allowed to settle.The clear supernatant solution turns the pH paper yellowish-orange.Which of the following would change the colour of this pH paper to greenish-blue?
The aqueous solution of which of the following substance is a good conductor of electricity?
The values of formic acid and acetic acid are respectively and . The ratio of acid strength of acids is
The acidic and basic strength of solution is based on _______________ ion concentration.
and are the values of four carboxylic acids respectively. What is the value of strongest carboxylic acid among them?
Which of the following is expected to be most highly ionised in water?
and HCl are all strong acids in aqueous solution. In glacial acetic acid medium, their acid strength is such that
Equimolar solutions of HF, HCOOH and HCN at 298 K have the values of as and respectively. What will be the order of their acidic strength?
The dissociation constant of two weak acids are and respectively. Their relative strength of identical concentration is-
The values of four carboxylic acids are and respectively. What is the value of strongest carboxylic acid among them?
The ratio of acid strength of and is about
Given of and of
Four species are listed below
1.
2.
3.
4.
Which one of the following is the correct sequence of their acid strength?
is weaker acid than . It is due to